August 03, 2021
The Orthopaedic Implant Company Launches FDA Cleared DRPx Wrist Fracture Plate
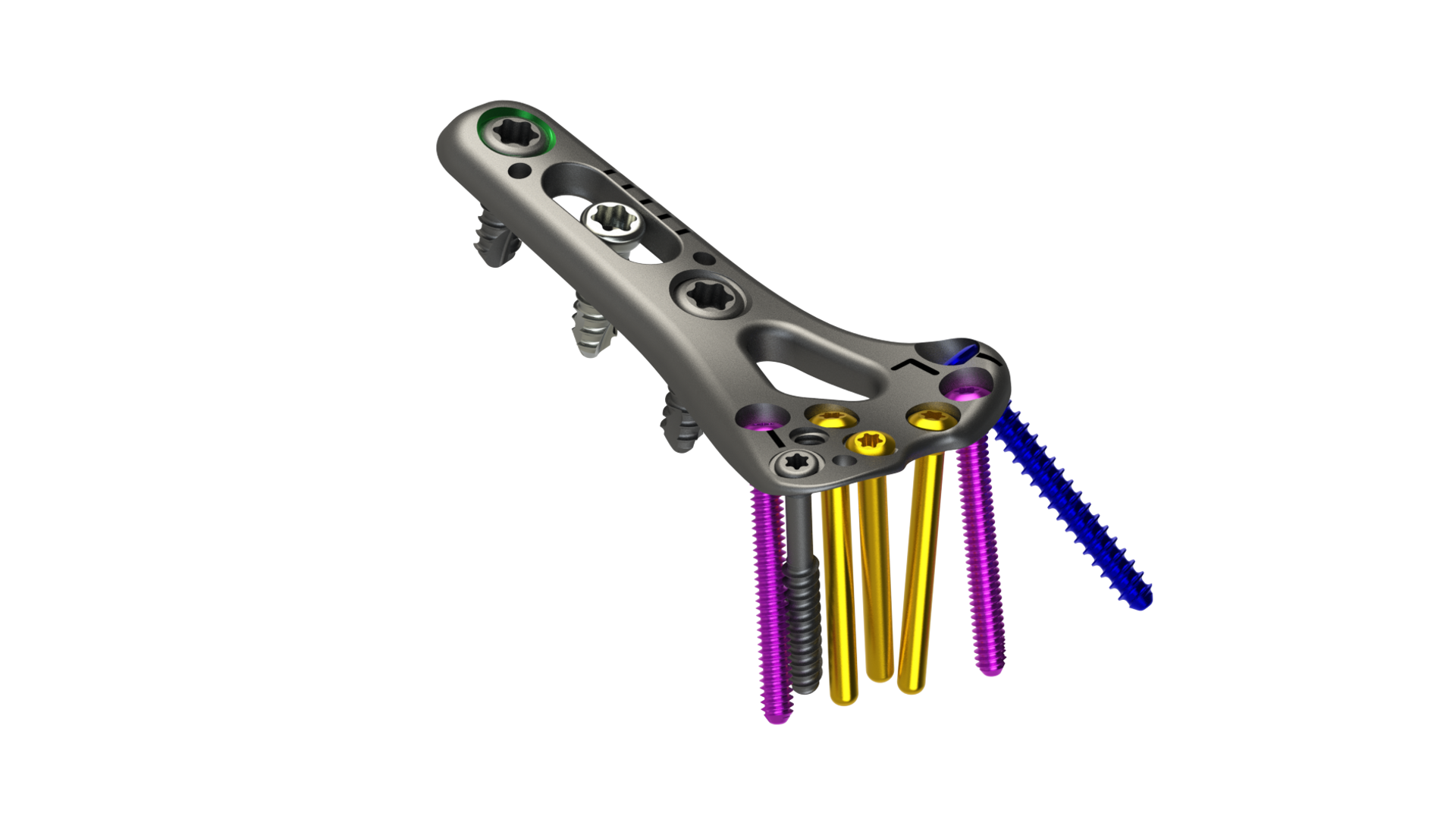
RENO, Nevada, August 4, 2021 – The Orthopaedic Implant Company today announced the FDA clearance and commercial launch of its wrist fracture plating technology – the DRPx System. DRPx is the only distal radius plating system that features an enhanced ergonomic design to meet the technique preferences of orthopaedic surgeons while significantly increasing cost savings to help improve the financial viability of ambulatory surgery centers (ASCs) and hospitals.
RENO, Nevada, August 3, 2021 – The Orthopaedic Implant Company today announced the FDA clearance and commercial launch of its wrist fracture plating technology – the DRPx System. DRPx is the only distal radius plating system that features an enhanced ergonomic design to meet the technique preferences of orthopaedic surgeons while significantly increasing cost savings to help improve the financial viability of ambulatory surgery centers (ASCs) and hospitals.
Distal radius fractures are the most common orthopaedic injury, comprising nearly 25% of all upper extremity fractures in the U.S. Because of the renewed focus on improving the efficiency of care delivery, the treatment of these injuries is increasingly moving to ASCs. However, the high costs associated with conventional distal radius plating systems create significant financial strain for surgery centers. DRPx provides ASCs with a solution that is designed to deliver exceptional clinical outcomes and value necessary to support surgery centers’ ongoing commitment to align on patient needs, sustain cultures driven by improvement, and create cost-savings that improve financial viability.
“Wrist fractures are one of the most common injuries treated by orthopedic surgeons. For those patients who benefit from surgical treatment, it is important to do so quickly, safely, and predictably. With the many barriers surgeons face in providing this type of care in today’s health care system, there has been a shift towards the outpatient surgical setting. Lowering the cost of internal fixation devices eliminates cost as the rate limiting step for successfully moving orthopedic trauma into the surgery center setting,” said Nikola Babovic, M.D., Reno Orthopedic Center, Reno, Nev. “The creation of a truly high value distal radius plate such as the DRPx is long overdue, as it will help surgery centers reduce the cost of care without sacrificing quality. These savings can be passed directly to patients, especially those who are underinsured or paying out of pocket for medical care.”
Designed with Type II anodized titanium, the DRPx features:
- Pre-contoured anatomic plates
- Low-profile plate and screw design to minimize soft tissue impingement
- Variable angle and fixed angle locking screws, locking pegs, and non-locking screws provide optionality for any technique
- Distal window for graft and/or fracture visualization
- Indicator lines for improved guidance and adjustment
- A full spectrum of targeting instrumentation that facilitates precise and fast drilling trajectories
“ A new standard needs to be set clinically and financially in orthopaedics, and that’s what we’re doing with DRPx,” said Itai Nemovicher, CEO of the Orthopaedic Implant Company. “DRPx can compete with the most feature-rich distal radius plates on the market. It is engineered to support exceptional clinical outcomes and reduce costs while providing surgeons with much-needed familiarity and flexibility when it comes to technique.”
DRPx is commercially available in the United States. For more information, please visit www.orthoimplantcompany.com or email info@orthoimplantcompany.com.
References
Corsino, Carlin B., et al. “Distal Radius Fractures.” Treasure Island (FL): StatPearls Publishing; 2021
