January 25, 2017
OIC Launches Intramedullary Tibial Nail
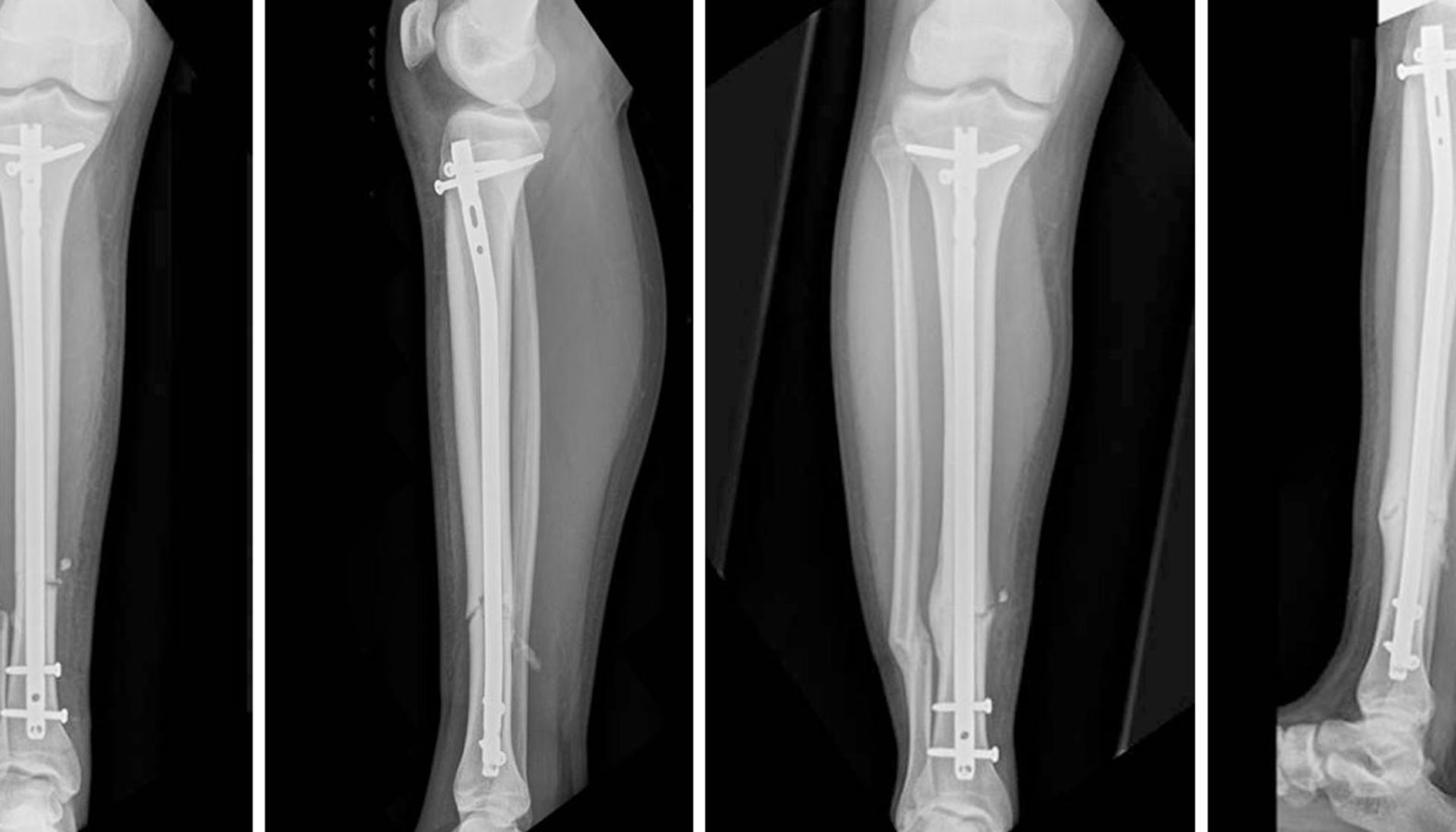
The Orthopaedic Implant Company (OIC), a medical device company that specializes in high-value orthopaedic implants, announced the launch of its Tibial Intramedullary Nailing System (https://web.archive.org/web/20190513224509/http://orthoimplantcompany.com/tibial-nail/) today.
January 24, 2017 (RENO, NV) – The Orthopaedic Implant Company (OIC), a medical device company that specializes in high-value orthopaedic implants, announced the launch of its Tibial Intramedullary Nailing System today.
The OIC Tibial Nail System is designed to stabilize fractures of the tibia. Strategically placed proximal and distal screw holes accommodate varying fracture patterns with a proximally-placed slotted hole that allows dynamization. The nail is available in diameters of 9mm through 11.5mm and lengths of 280mm to 420mm and features 5.0mm diameter cross-lock screws for optimal implant fixation.
The OIC Tibial Nail is part of OIC’s high value IM Nail System and uses the same instrumentation as all IM nails from OIC – decreasing inventory burdens and costs while increasing intra-operative efficiencies. It is the third nail released in the last twelve months from the company that address long bone fractures. “We are very proud to offer the addition of the OIC Tibial Nail to our high value implant portfolio,”, said Todd Martens, VP of Product Design and Customer Experience . “We’ve designed the entire IM Nail System to meet the demands of value based healthcare; a rapidly growing trend.”
